how do metal carbonates and metal hydrogen carbonates react with acids ? give their chemical equations . - Brainly.in
6.10 g metal carbonate on heating produces 5.6 gram of metal oxide,then equivalent weight of metal carbonate is?

Question Video: Recalling the Products of the Reaction between a Metal Carbonate and an Acid | Nagwa

Chemistry – Metal carbonate and hygrodencarbonates - Acids, bases and salts - Part 2 -English - YouTube
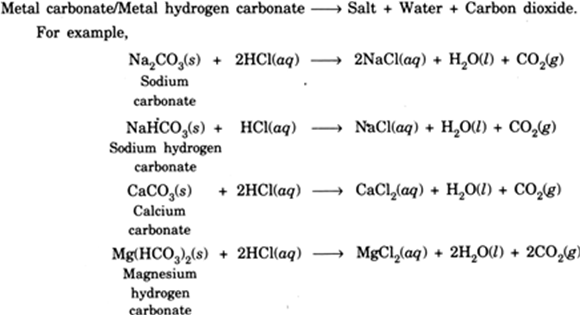
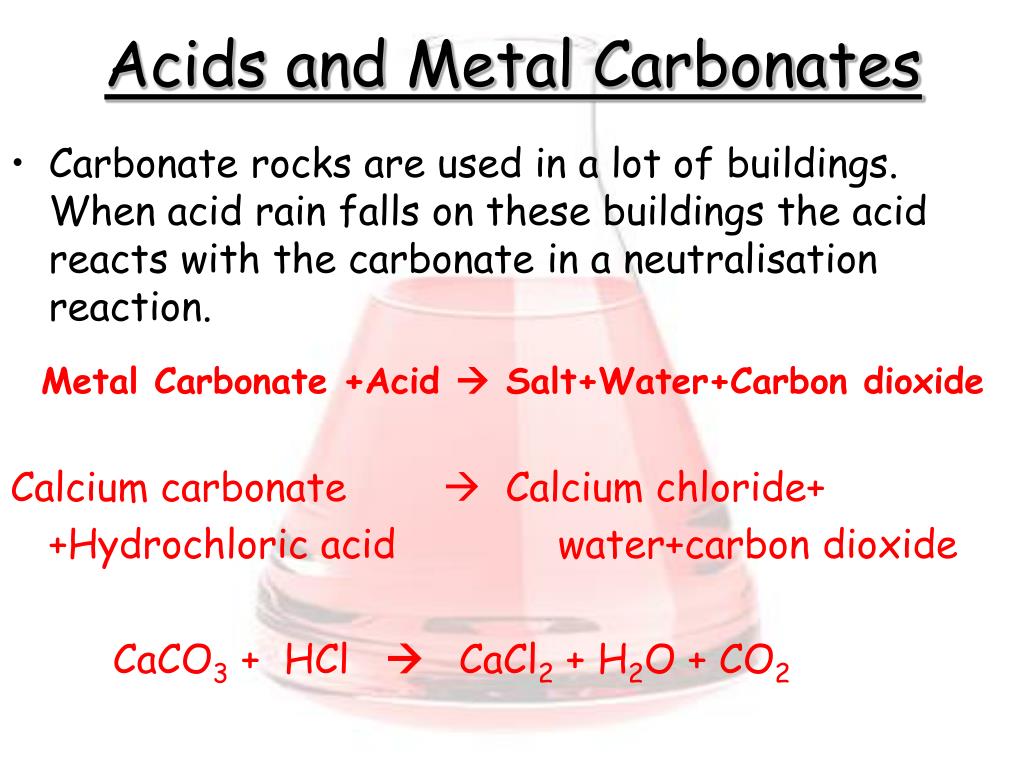
Name the gas evolved when a metal carbonate or metal hydrogen carbonate reacts with acids. Explain the chemical reaction. from Science Acids, Bases and Salts Class 10 Haryana Board - English Medium


![MCQ] Which of the statements is not correct? All metal oxides react MCQ] Which of the statements is not correct? All metal oxides react](https://d1avenlh0i1xmr.cloudfront.net/0146fde4-9748-4104-875a-ccbefb146ee1/reaction-of-metal-carbonate-with-acid---teachoo-01.jpg)


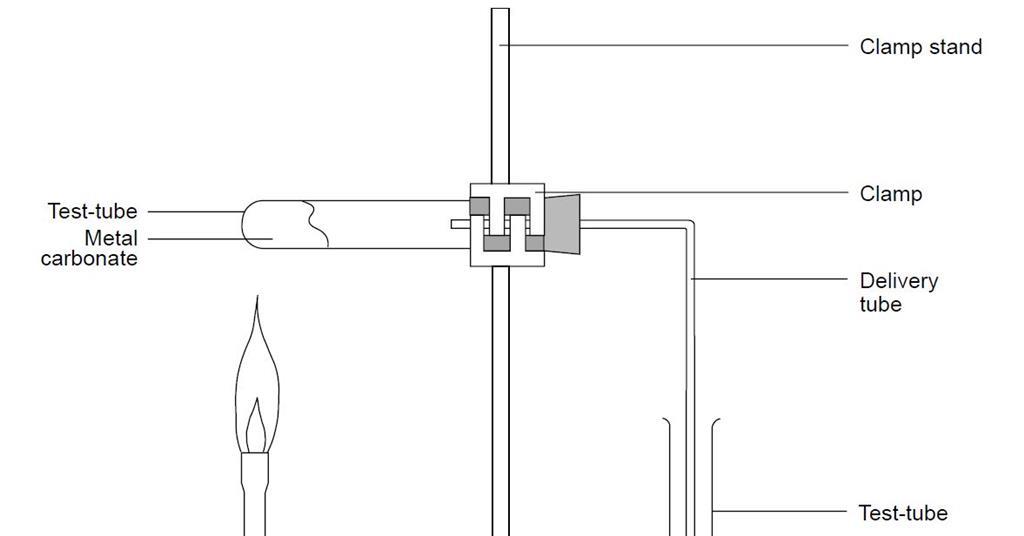
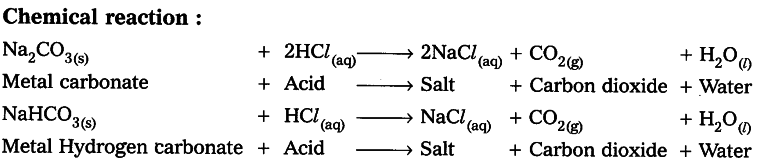
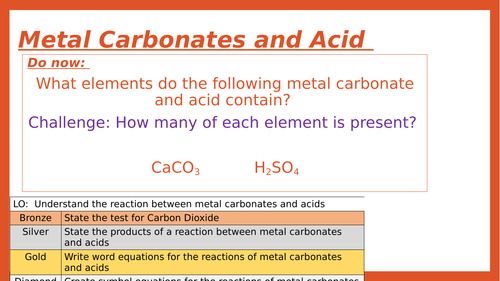


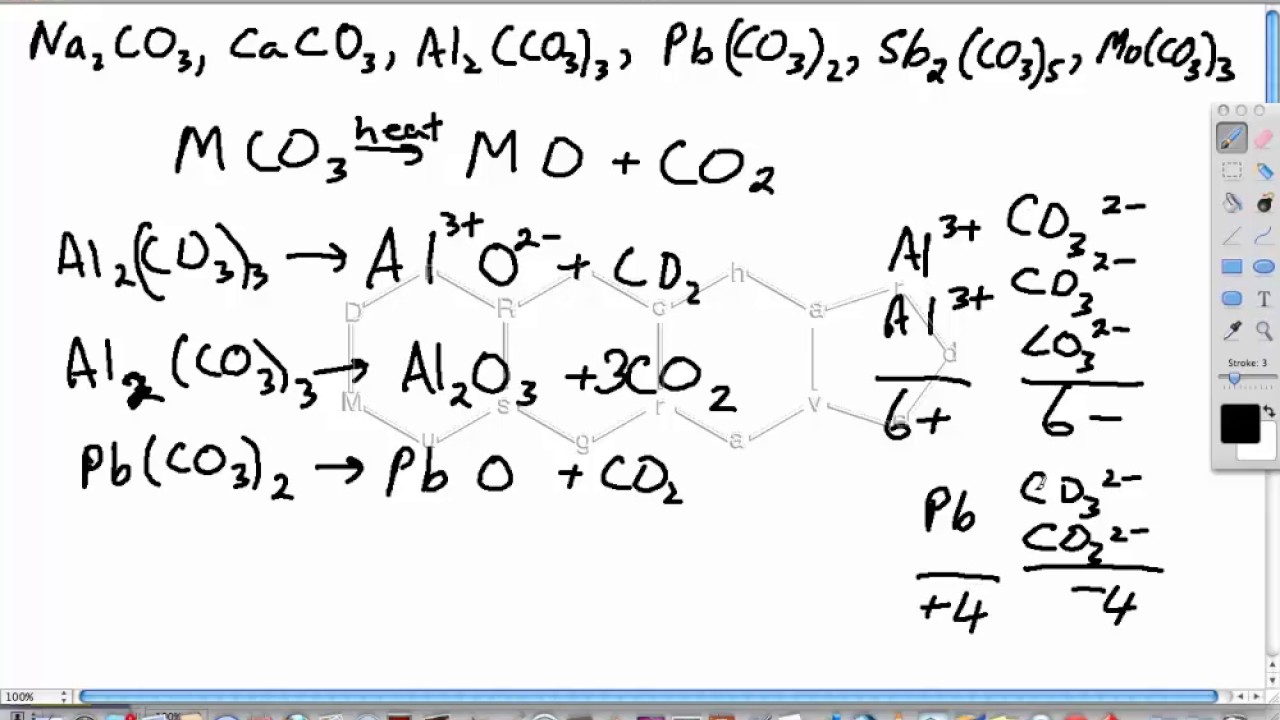
.png)